Our Science
Cell-targeted lipid nanoparticle (ctLNP): a breakthrough technology for targeted delivery
Our proprietary ctLNP technology enables selective access to T cells and precise target inhibition by siRNA to selectively modulate T cells , blocking autoimmune tissue damage while sparing the broader immune system.
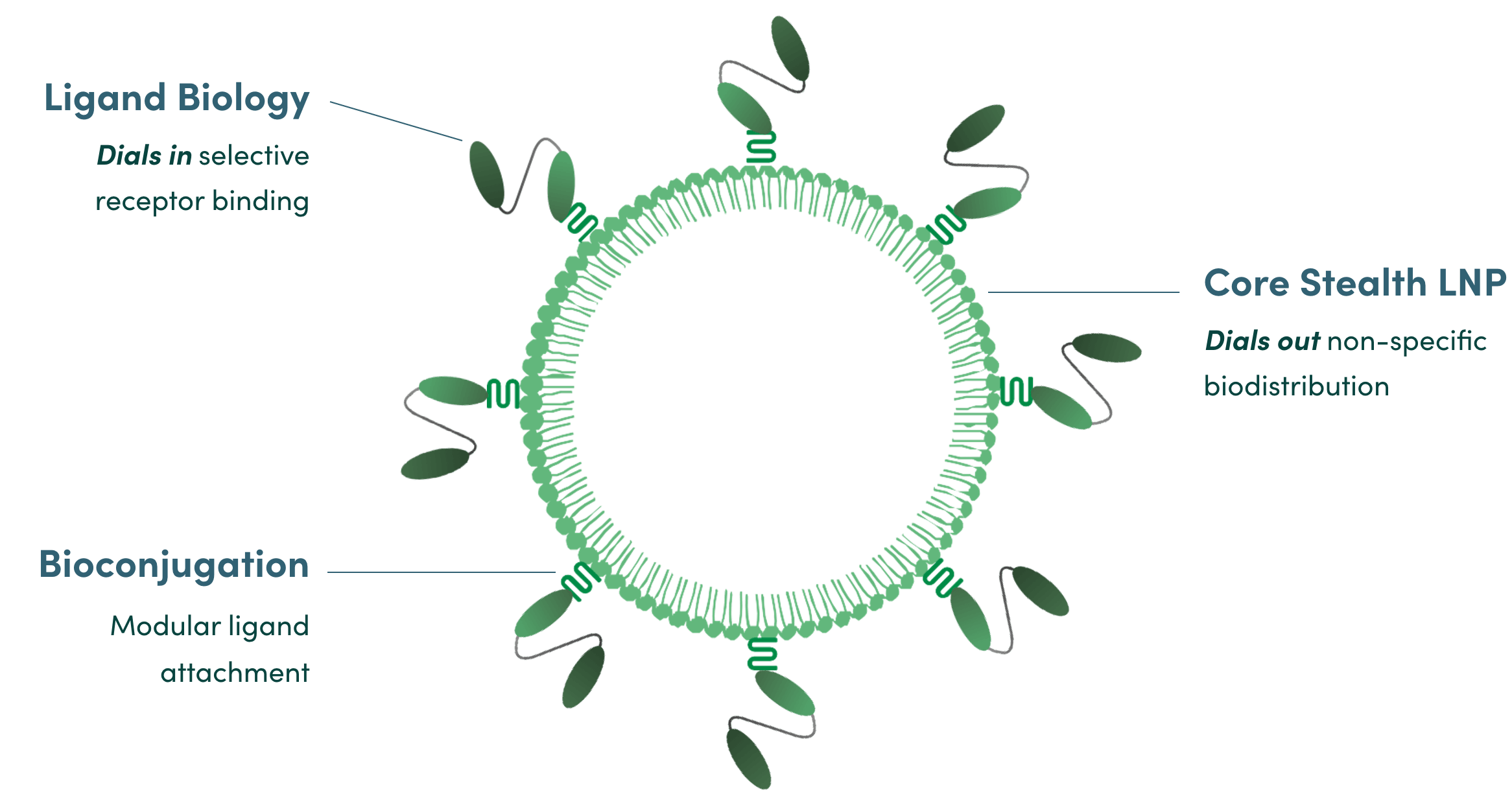
Our proprietary stealth technology prevents our LNP from interacting with serum-binding proteins and reduces clearance by the liver and spleen to less than 1%, enabling to superior potency & selectivity.
Designed for modularity, we can swap ligands to target new cell types, and our ctLNP is compatible with a wide array of payloads
Silences targets in T cells , not in other cell types, because uptake only occurs in cells that express our target receptor
Unlocking undruggable targets in T cells for the first time
Our ctLNP selectively reaches T cells and is specifically engineered to increase cytoplasmic exposure, enabling functional siRNA delivery. Direct conjugates can’t deliver effectively to T cells due to low cytoplasmic exposure.
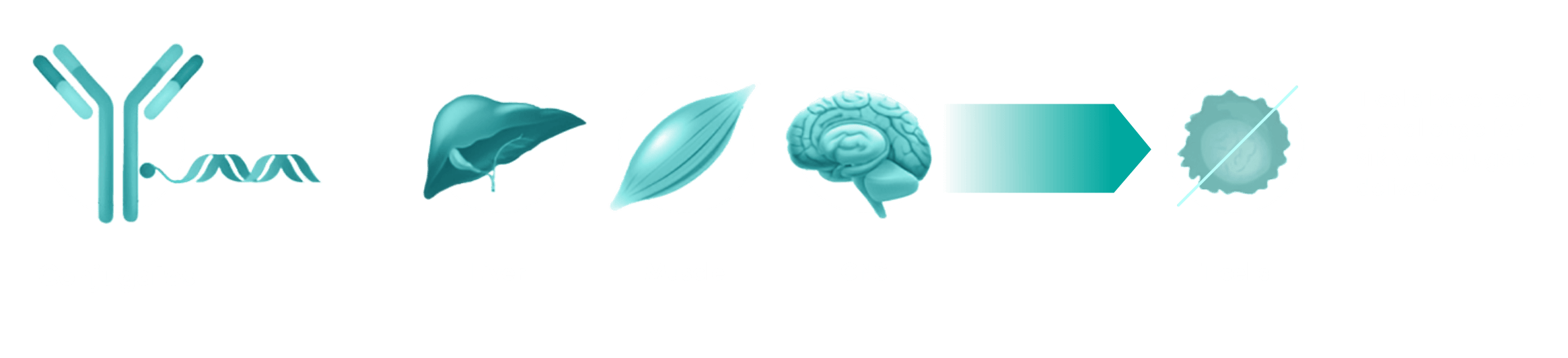

Novel access to T cells unlocks a series of targets that have the potential to change how T cells activate, differentiate, infiltrate, and damage tissues in T cell–driven autoimmune diseases.
Our selective delivery allows us to inhibit targets in T cells while sparing critical function in other cell types. Additionally, siRNA enables us to access difficult-to-drug targets with selectivity and durability, as many of these targets are not accessible from the cell surface where antibodies function.
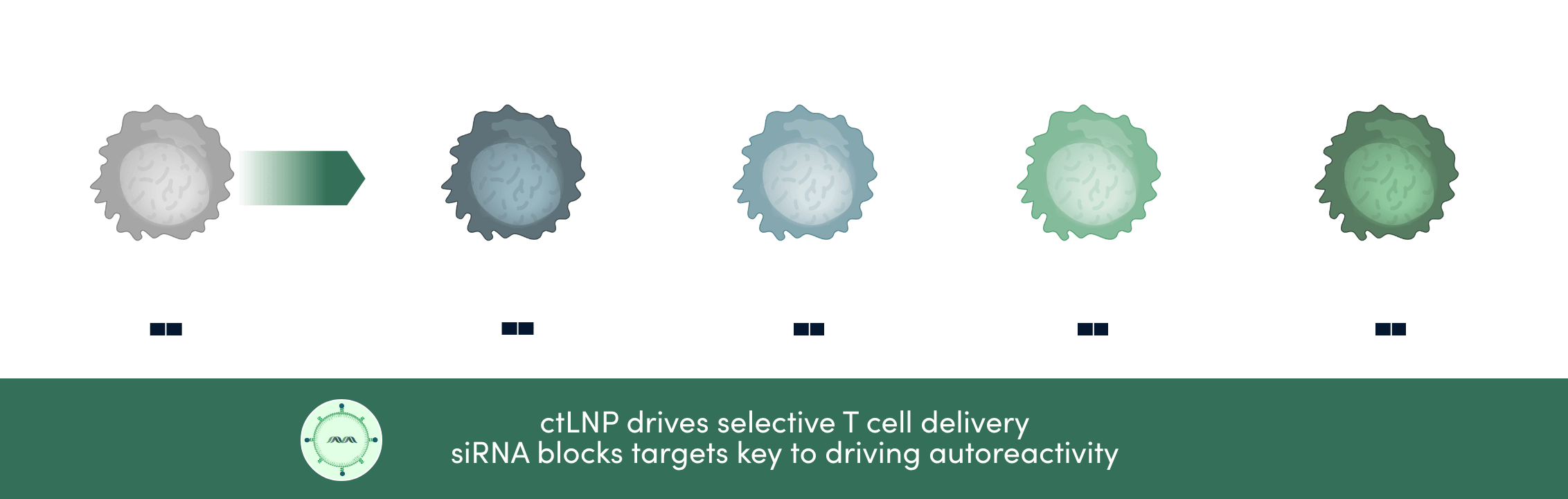
Modulating T cell activity to combat autoimmune diseases
Leveraging our ctLNP-siRNA technology, we’re uniquely positioned to address a range of poorly drugged or undruggable T cell targets.